Water-soluble tin(IV) porphyrins as photosensitisers for artificial photosynthesis
 |
Anne-Marie Manke1, Karen Geisel2 and Philipp Kurz1
1 Christian-Albrechts-University Kiel, Institute for Inorganic Chemistry, Max-Eyth-Str. 2, 24118 Kiel, Germany and
2 RWTH Aachen University, Institute for Physical Chemistry, Landoltweg 2, 52056 Aachen, Germany
e-mail: amanke@ac.uni-kiel.de
URL: http://www.ac.uni-kiel.de/phkurz
|
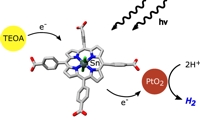 |
One concept for sustainable energy generation is light-driven water-splitting into hydrogen and oxygen. Such schemes are inspired by basic principles of photo-synthesis, the process where solar energy is converted into chemically stored energy in nature. In vivo, chlorophyll molecules act as light absorbing molecules and electron transfer agents. They generate both the high oxidising potentials for water-oxidation and the strong reducing agents for NADP+-reduction.
We study the water-soluble tin(IV) porphyrins dichlorido-5,10,15,20- tetrakis(p-carb-oxyphenyl)-porphyrinato-tin(IV) (SnTPPC) and dichlorido-5,10,15,20- tetrakis(p-sulfo-phenyl)-porphyrinato-tin(IV) (SnTPPS) as possible mimics for chlorophyll dyes. The compounds show favourable redox potentials to act as photosensitisers in an artificial system for light-driven water-splitting. We tested the ability of these synthetic porphyrins to act as photosensitisers in photocatalytic model systems and especially detected promising rates for light-induced H2 formation in a photocatalytic system developed by Krüger and Fuhrhop.[1] Details on possible mechanisms, the reaction kinetics, photosensitiser stability and the use of different electron donors will be presented.



