
Department of Chemistry and Biochemistry, Arizona State University, Tempe, AZ, U.S.A.
e-mail: gghirlanda@asu.edu
 |
Anindya Roy, Mathieu Walthers, and Giovanna Ghirlanda
Department of Chemistry and Biochemistry, Arizona State University, Tempe, AZ, U.S.A. e-mail: gghirlanda@asu.edu |
 |
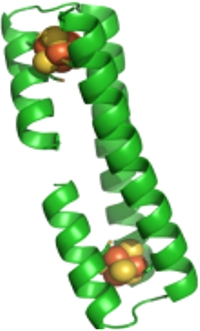
The long-term goal of this project is to develop a reducing catalyst capable of generating molecular hydrogen from aqueous protons. We are using a bioinspired approach, which recapitulates all components of the natural hydrogenases in an integrated system: a catalytic center, the activity of which is modulated by the protein matrix in which it is embedded; a molecular wire, which forms the conduit to transfer electrons to the active site; and a designed protein-electrode interface, which connects the catalyst to the rest of the device. In turns, the catalyst and the molecular wire are embedded in a designed protein scaffold, such that the spatial distribution of the cofactors and their distance from each other can be controlled with high precision. Towards this goal we designed a family of coiled-coil helical proteins, which we functionalize with organometallic active sites. As coiled coil are repeat proteins, we can now control the distance between cofactors by translating the binding site along the coiled-coil axis, thus designing molecular wires that we interface with other functional protein modules via specific protein-protein interactions. By introducing additional organometallic centers into each three helix bundle, we aim to reconstruct reaction chains to convert the collected energy into a fuel, mimicking the function of natural photosystems. Exploiting the symmetry of designed protein, we recently designed a nanowire that contains two iron sulfur clusters in the core of the closed dimeric three helix bundle DSD at tunable distances, DSD-[Fe4S4]. This construct demonstrates that we can use symmetry to incorporate two clusters at defined distances. We also demonstrated direct incorporation into the helical framework of a an intact (µ-SRS)[Fe(CO)3]2 cluster, covalently anchored to the protein. Current work is focused on directly interfacing the [Fe4S4] cluster with the H-cluster of hydrogenase.
This work is part of the Biofuel Center at Arizona State University, funded by the Department of Energy.