
Biomedical Research Center/FB15, Philipps University, Hans-Meerwein-Straße, 35043 Marburg, Germany
 |
Yann Geisselbrecht, Stephan Kiontke, and Lars-Oliver Essen
Biomedical Research Center/FB15, Philipps University, Hans-Meerwein-Straße, 35043 Marburg, Germany |
 |
Cryptochromes and photolyases (PHL) are structurally related and ubiquitously occurring blue light receptors. To enhance their absorbance cross section in the blue region of the visible light spectrum the members of the photolyase/cryptochrome family utilise a variety of aromatic chromophores, namely methenyl-tetrahydrofolate (MTHF in E. coli CPD I PHL, A. thaliana Cry3), flavin-mononucleotide (FMN in T. thermophilus PHL), flavin-adenin-dinucleotide (FAD in S. tokodaii PHL) and 8-hydroxydeazaflavin (8-HDF in M. mazei PHL, D. melanogaster 6-4 PHL).
The latest member of these antenna chromophores was identified as 6,7-dimethyl-8-ribityl-lumazine (DLZ) bound to cryptochrome B from Rhodobacter sphaeroides. CryB belongs to the distinct CryPro clade inside the photolyase/cryptochrome family and is the first member of this protein family additionally harbouring a [4Fe-4S] cluster.
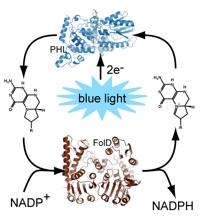
A challenging task is the in vivo reconstitution of apo-photolyases with their antenna, if the expression host lacks the ability to produce the adequate molecule. To give E. coli the ability to produce 8-HDF the F0-synthase of S. coelicolor was coexpressed, and this setup was tested for the in vivo reconstitution of various photolyases. Furthermore, this approach gives the opportunity to produce therefrom derived deazaflavins, like deaza-FAD, in E. coli and directly incorporate them into photolyases and therefore massively alter their spectroscopic and redox behaviour.
At least one antenna chromophore, MTHF, is not only an innocent "light harvesting molecule" but can also act as an electron acceptor in a process named photobleaching. The first product of this reaction is methylene-THF, with low affinity to the photolyase. This product can be easily reoxidized by folate-dehydrogenases in the presence of NADP+. The resulting in vitro photocycle pumps electrons from a donor to NADPH as storage form.