
Lehrstuhl für Organische Chemie 1, Department Chemie, Technische Universität München, Garching, Germany
e-mail: thorsten.bach@ch.tum.de
 |
Thorsten Bach
Lehrstuhl für Organische Chemie 1, Department Chemie, Technische Universität München, Garching, Germany e-mail: thorsten.bach@ch.tum.de |
 |
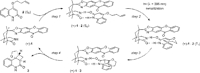
Hydrogen bonds to the lactam unit of 1,5,7-trimethyl-3-azabicyclo[3.3.1]nonan-2-ones or of octahydro-1H-4,7-methanoisoindol-1-ones allow to position a given substrate in a distinct chiral environment, in which enantiotopic face differentiation is possible. This fact has been extensively employed by our group for enantioselective photochemical and radical reactions in the presence of a stoichiometric, chiral 1,5,7-trimethyl-3-azabicyclo[3.3.1]nonan-2-one.[1] By attaching to the position 7 of the heterocyclic skeleton a sensitizing fragment, as depicted for xanthone (+)-1, it has been possible to perform reactions of this type in a catalytic fashion.[2-4] The catalytic cycle for the conversion of substrate 2 --> 3 (75% yield, 90% ee with 10 mol-% catalyst) shows that several requirements need to be fulfilled for such a reaction to be successful.
The presentation discusses the background of the above-mentioned studies and provides the latest results of our research efforts.